|
Contributed by Dr. Jochen Utikal
Sample preparation I
FISH on touch preparations of unfixed tumour tissue/cell smears/cytospins of cultured or blood cells
- Fix touch preparations of unfixed tumour tissue/cell smears/cytospins of cultured or blood cells in 4% paraformaldehyde in 1x PBS for 20 minutes.
- Wash in 3x PBS, 1x PBS and 1x PBS (5 minutes each)
- Dehydrate in ethanol (30%, 60%, 80%, 95%, 100%) (2 minutes each) slides can be stored at –80°C or continue with the FISH protocol
Sample preparation II
FISH on paraffin embedded tissue sections
- Fix paraffin embedded tissue sections on positive charged slides
- Deparaffinize tissue sections in xylene twice for 10 minutes and dehydrate with 100% ethanol
- Pre-treat with the Vysis paraffin pretreatment kit (Vysis Inc, Downers Grove, IL). Continue with FISH protocol
Sample preparation III
FISH on Metaphase spreads
- Perform metaphase spreads according to standard protocols and air dry. Placement of slides on a 45°C for 1 day can increase results. Continue with FISH protocol
FISH protocol
- Incubate slides with RNase (100µg/ml) at 37°C for 1 hour.
- Incubate slides in 2x SSC at 75°C for 15 min.
- Digest in pepsin solution (4 mg/ml in 0.9% NaCl, pH 1.5) for 15 min at 37°C.
- Rinse in 2x SSC at room temperature for 5 min and air dry at 37°C.
- Prepare the hybridisation mixture (mix directly labelled fluorescent DNA probes e.g. from Vysis, Downers Grove, IL, USA with the appropriate hybridisation buffer (containing probe depending concentrations of dextran sulfate, formamide and SCC (pH 7.0) (available from Vysis, Downers Grove). Dual or poly probe hybridisation can be performed easily using probes labelled with different fluorochromes.
- Add hybridisation mixture to a diamond tipped scribe marked area on the slide and apply a coverslip immediately upon placing the probe on the slide.
- Denature probes and target DNA simultaneously on the slide in an 75 °C oven for 3 min (touch preparations of unfixed tumour tissue/cell smears/cytospins of cultured or blood), in an 75°C oven for 4 min (paraffin embedded tissue sections) and in an 72°C oven for 2 min (metaphase spreads).
- Seal coverslip with rubber cement.
- Incubate slide at 42°C in a humidified box overnight.
- Remove rubber cement seal and coverslip.
- Wash in 1.5 M Urea/ 0.1x SSC at 45 °C for 30 min.
- Wash in 2x SSC at room temperature for 2 min.
- Counter stain with 4´,6-diamidino-2-phenylindole and antifade compound p-phenylenediamine.
- Analyse with a fluorescence microscope equipped with appropriate filters.
Examples
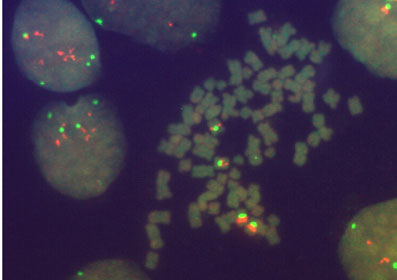
FISH performed on a metaphase spread of cell line A431 with probes for the Cyclin D1 gene locus at 11q13 (orange signals) and the centromeric region of chromosome 11 (green signals)
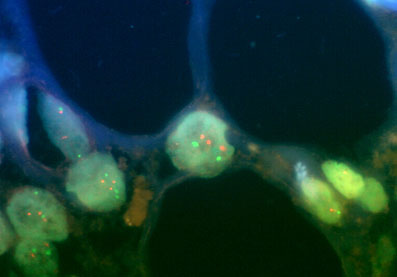
FISH performed on a paraffin embedded tissue section of a malignant melanoma with probes for the Cyclin D1 gene locus at 11q13 (orange signals) and the centromeric region of chromosome 11 (green signals)

|