|
Contributed by Dr. Alexei Gratchev
We use this protocol since years for different types of mammalian cells. The cells should be grown in a 90mm Petri dish to monolayer. Please note, that this protocol does not substitute the handbook provided by Qiagen and was thought to provide a quick reference.
Important notes before you start
- Always wear gloves while working with RNA.
- Change your gloves as often as you can.
- Clean your bench with RNase-Erase or similar RNase killer.
- Use filtered pipet tips if possible.
Protocol
- Prepare the lysis buffer (700 µl per 90 mm dish) by adding 10 µl of b-mercaptoethanol to each 1 ml of the RLT buffer. Mix well.
- Aspirate culture medium from the Petri dish.
- Hold the dish inclined for 30 sec, aspirate the rests of the culture medium.
- Pipet 600µl of the lysis buffer onto the dish, distribute the buffer evenly by rocking the dish.
- Using the cell scraper collect all the lysis buffer on one side of the dish.
- Transfer the lysate into a 2 ml tube.
- Homogenize the lysate by passing it 10 times through a 20G needle.
- To homogenized lysate add 1 volume (600 µl) of 70% ethanol.
- Vortex thoroughly.
- Transfer 700 µl of the sample to a RNeasy column, placed in provided 2 ml tube.
- Centrifuge 1 min at 13000 rpm.
- Discard flow-through.
- Repeat step 10 by transferring the rest of sample to the column.
- Centrifuge 1 min at 13000 rpm.
- Discard flow-through.
- Apply 600 µl of RW1 buffer to the column.
- Centrifuge 1 min at 13000 rpm.
- Discard flow-through.
- Apply 500 µl of buffer RPE to the column.
- Centrifuge 1 min at 13000 rpm.
- Place RNeasy column in a fresh 2 ml tube (provided).
- Apply 500 µl of buffer RPE to the column.
- Centrifuge 3 min at 13000 rpm.
- Place RNeasy column in a collection tube.
- Apply 50 µl of hot (60-70°C) RNase free water directly to the membrane.
- Incubate 1 min.
- Centrifuge 1 min at 13000 rpm.
- Apply another 50 µl of hot (60-70°C) RNase free water to the membrane.
- Centrifuge 3 min at 13000 rpm.
- Transfer eluate into a fresh RNase free tube (not provided), determine the concentration photometrically.
- The quality of the obtained RNA samples may be analyzed on a standard 1% agarose, 1x TAE gel (figure 1).
- Store isolated samples at –80°C.
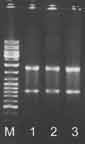
Figure 1. M - DNA ladder, 1-3 - RNA samples
Links

|